JavaScript is disabled for your browser. Some features of this site may not work without it.
Buscar en RiuNet
Listar
Mi cuenta
Estadísticas
Ayuda RiuNet
Admin. UPV
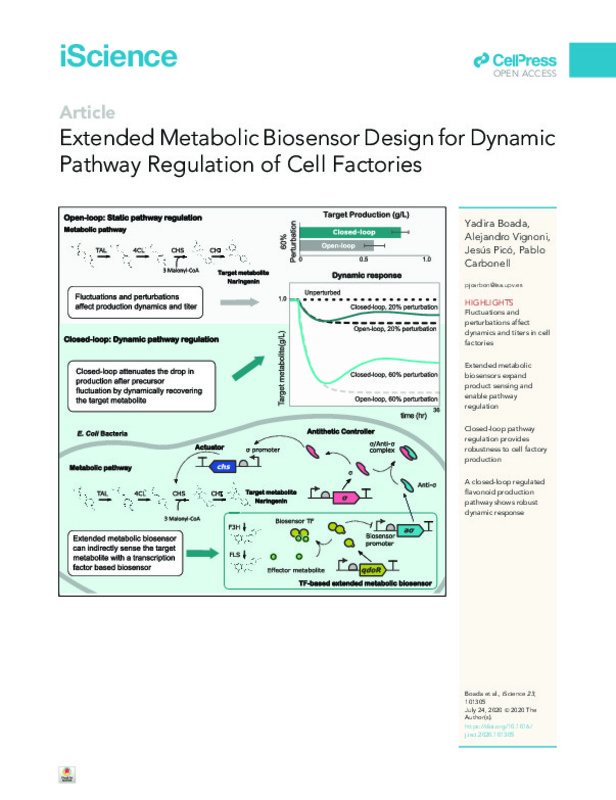
Extended metabolic biosensor design for dynamic pathway regulation of cell factories
Mostrar el registro sencillo del ítem
Ficheros en el ítem
| dc.contributor.author | Boada-Acosta, Yadira Fernanda
|
es_ES |
| dc.contributor.author | Vignoni, Alejandro
|
es_ES |
| dc.contributor.author | Picó, Jesús
|
es_ES |
| dc.contributor.author | Carbonell, Pablo
|
es_ES |
| dc.date.accessioned | 2021-07-22T03:33:46Z | |
| dc.date.available | 2021-07-22T03:33:46Z | |
| dc.date.issued | 2020-07-24 | es_ES |
| dc.identifier.uri | http://hdl.handle.net/10251/169743 | |
| dc.description.abstract | [EN] Transcription factor-based biosensors naturally occur in metabolic pathways to maintain cell growth and to provide a robust response to environmental fluctua-tions. Extended metabolic biosensors, i.e., the cascading of a bio-conversion pathway and a transcription factor (TF) responsive to the downstream effector metabolite, provide sensing capabilities beyond natural effectors for implement-ing context-aware synthetic genetic circuits and bio-observers. However, the engineering of such multi-step circuits is challenged by stability and robustness issues. In order to streamline the design of TF-based biosensors in metabolic pathways, here we investigate the response of a genetic circuit combining a TF-based extended metabolic biosensor with an antithetic integral circuit, a feed-back controller that achieves robustness against environmental fluctuations. The dynamic response of an extended biosensor-based regulated flavonoid pathway is analyzed in order to address the issues of biosensor tuning of the regulated pathway under industrial biomanufacturing operating constraints. | es_ES |
| dc.description.sponsorship | This work is partially supported by grant MINECO/AEI and EU DPI2017-82896-C2-1-R. P.C. acknowledges support from the Universitat Politecnica de Valencia Talento Programme. | es_ES |
| dc.language | Inglés | es_ES |
| dc.publisher | Elsevier (Cell Press) | es_ES |
| dc.relation.ispartof | iScience | es_ES |
| dc.rights | Reconocimiento (by) | es_ES |
| dc.subject.classification | INGENIERIA DE SISTEMAS Y AUTOMATICA | es_ES |
| dc.title | Extended metabolic biosensor design for dynamic pathway regulation of cell factories | es_ES |
| dc.type | Artículo | es_ES |
| dc.identifier.doi | 10.1016/j.isci.2020.101305 | es_ES |
| dc.relation.projectID | info:eu-repo/grantAgreement/AEI/Plan Estatal de Investigación Científica y Técnica y de Innovación 2013-2016/DPI2017-82896-C2-1-R/ES/DISEÑO, CARACTERIZACION Y AJUSTE OPTIMO DE BIOCIRCUITOS SINTETICOS PARA BIOPRODUCCION CON CONTROL DE CARGA METABOLICA/ | es_ES |
| dc.rights.accessRights | Abierto | es_ES |
| dc.contributor.affiliation | Universitat Politècnica de València. Departamento de Ingeniería de Sistemas y Automática - Departament d'Enginyeria de Sistemes i Automàtica | es_ES |
| dc.description.bibliographicCitation | Boada-Acosta, YF.; Vignoni, A.; Picó, J.; Carbonell, P. (2020). Extended metabolic biosensor design for dynamic pathway regulation of cell factories. iScience. 23(7):1-25. https://doi.org/10.1016/j.isci.2020.101305 | es_ES |
| dc.description.accrualMethod | S | es_ES |
| dc.relation.publisherversion | https://doi.org/10.1016/j.isci.2020.101305 | es_ES |
| dc.description.upvformatpinicio | 1 | es_ES |
| dc.description.upvformatpfin | 25 | es_ES |
| dc.type.version | info:eu-repo/semantics/publishedVersion | es_ES |
| dc.description.volume | 23 | es_ES |
| dc.description.issue | 7 | es_ES |
| dc.identifier.eissn | 2589-0042 | es_ES |
| dc.identifier.pmid | 32629420 | es_ES |
| dc.identifier.pmcid | PMC7334618 | es_ES |
| dc.relation.pasarela | S\414464 | es_ES |
| dc.contributor.funder | Universitat Politècnica de València | es_ES |
| dc.contributor.funder | Agencia Estatal de Investigación | es_ES |
| dc.description.references | Agrawal, D. K., Dolan, E. M., Hernandez, N. E., Blacklock, K. M., Khare, S. D., & Sontag, E. D. (2020). Mathematical Models of Protease-Based Enzymatic Biosensors. ACS Synthetic Biology, 9(2), 198-208. doi:10.1021/acssynbio.9b00279 | es_ES |
| dc.description.references | Arnold, F. H. (2017). Directed Evolution: Bringing New Chemistry to Life. Angewandte Chemie International Edition, 57(16), 4143-4148. doi:10.1002/anie.201708408 | es_ES |
| dc.description.references | Boada, Y., Vignoni, A., & Picó, J. (2017). Engineered Control of Genetic Variability Reveals Interplay among Quorum Sensing, Feedback Regulation, and Biochemical Noise. ACS Synthetic Biology, 6(10), 1903-1912. doi:10.1021/acssynbio.7b00087 | es_ES |
| dc.description.references | Boada, Y., Vignoni, A., & Picó, J. (2017). Multi-objective optimization for gene expression noise reduction in a synthetic gene circuit * *This work is partially supported by Spanish government and European Union (FEDER-CICYT DPI2014-55276-C5-1). Y.B. thanks grant FPI/2013-3242 of Universitat Politècnica de València, and also thanks the support from the Ayudas para movilidad dentro del Programa para la Formación de Personal Investigador (FPI) de la UPV para estancias 2016. A.V. thanks the Max Planck Society, the CSBD and the MPI-CBG. The authors are grateful to Prof. Dr. Ivo F. Sbalzarini for hosting Y.B in the MOSAIC Group for a research stay, also to Pietro Incadorna from the MOSAIC Group at CSBD for his help in the parallel algorithm implementation, and to Dr. Gilberto Reynoso-Meza from the PPGEPS at Pontifícia Universidade Católica do Paraná for his always helpful comments regarding the MOOD. IFAC-PapersOnLine, 50(1), 4472-4477. doi:10.1016/j.ifacol.2017.08.376 | es_ES |
| dc.description.references | Boada, Y., Vignoni, A., & Pico, J. (2020). Multiobjective Identification of a Feedback Synthetic Gene Circuit. IEEE Transactions on Control Systems Technology, 28(1), 208-223. doi:10.1109/tcst.2018.2885694 | es_ES |
| dc.description.references | Briat, C., Gupta, A., & Khammash, M. (2016). Antithetic Integral Feedback Ensures Robust Perfect Adaptation in Noisy Biomolecular Networks. Cell Systems, 2(1), 15-26. doi:10.1016/j.cels.2016.01.004 | es_ES |
| dc.description.references | Briat, C., & Khammash, M. (2018). Perfect Adaptation and Optimal Equilibrium Productivity in a Simple Microbial Biofuel Metabolic Pathway Using Dynamic Integral Control. ACS Synthetic Biology, 7(2), 419-431. doi:10.1021/acssynbio.7b00188 | es_ES |
| dc.description.references | Carbonell, P., Jervis, A. J., Robinson, C. J., Yan, C., Dunstan, M., Swainston, N., … Scrutton, N. S. (2018). An automated Design-Build-Test-Learn pipeline for enhanced microbial production of fine chemicals. Communications Biology, 1(1). doi:10.1038/s42003-018-0076-9 | es_ES |
| dc.description.references | Carbonell, P., Parutto, P., Baudier, C., Junot, C., & Faulon, J.-L. (2013). Retropath: Automated Pipeline for Embedded Metabolic Circuits. ACS Synthetic Biology, 3(8), 565-577. doi:10.1021/sb4001273 | es_ES |
| dc.description.references | Ceroni, F., Boo, A., Furini, S., Gorochowski, T. E., Borkowski, O., Ladak, Y. N., … Ellis, T. (2018). Burden-driven feedback control of gene expression. Nature Methods, 15(5), 387-393. doi:10.1038/nmeth.4635 | es_ES |
| dc.description.references | Chae, T. U., Choi, S. Y., Kim, J. W., Ko, Y.-S., & Lee, S. Y. (2017). Recent advances in systems metabolic engineering tools and strategies. Current Opinion in Biotechnology, 47, 67-82. doi:10.1016/j.copbio.2017.06.007 | es_ES |
| dc.description.references | Chen, X., & Liu, L. (2018). Gene Circuits for Dynamically Regulating Metabolism. Trends in Biotechnology, 36(8), 751-754. doi:10.1016/j.tibtech.2017.12.007 | es_ES |
| dc.description.references | Cheng, F., Tang, X.-L., & Kardashliev, T. (2018). Transcription Factor-Based Biosensors in High-Throughput Screening: Advances and Applications. Biotechnology Journal, 13(7), 1700648. doi:10.1002/biot.201700648 | es_ES |
| dc.description.references | Choi, J. H., Keum, K. C., & Lee, S. Y. (2006). Production of recombinant proteins by high cell density culture of Escherichia coli. Chemical Engineering Science, 61(3), 876-885. doi:10.1016/j.ces.2005.03.031 | es_ES |
| dc.description.references | Delépine, B., Libis, V., Carbonell, P., & Faulon, J.-L. (2016). SensiPath: computer-aided design of sensing-enabling metabolic pathways. Nucleic Acids Research, 44(W1), W226-W231. doi:10.1093/nar/gkw305 | es_ES |
| dc.description.references | Dinh, C. V., Chen, X., & Prather, K. L. J. (2020). Development of a Quorum-Sensing Based Circuit for Control of Coculture Population Composition in a Naringenin Production System. ACS Synthetic Biology, 9(3), 590-597. doi:10.1021/acssynbio.9b00451 | es_ES |
| dc.description.references | Doong, S. J., Gupta, A., & Prather, K. L. J. (2018). Layered dynamic regulation for improving metabolic pathway productivity inEscherichia coli. Proceedings of the National Academy of Sciences, 115(12), 2964-2969. doi:10.1073/pnas.1716920115 | es_ES |
| dc.description.references | Evans, C. R., Kempes, C. P., Price-Whelan, A., & Dietrich, L. E. P. (2020). Metabolic Heterogeneity and Cross-Feeding in Bacterial Multicellular Systems. Trends in Microbiology, 28(9), 732-743. doi:10.1016/j.tim.2020.03.008 | es_ES |
| dc.description.references | Gao, C., Xu, P., Ye, C., Chen, X., & Liu, L. (2019). Genetic Circuit-Assisted Smart Microbial Engineering. Trends in Microbiology, 27(12), 1011-1024. doi:10.1016/j.tim.2019.07.005 | es_ES |
| dc.description.references | Goldberg, A. P., Szigeti, B., Chew, Y. H., Sekar, J. A., Roth, Y. D., & Karr, J. R. (2018). Emerging whole-cell modeling principles and methods. Current Opinion in Biotechnology, 51, 97-102. doi:10.1016/j.copbio.2017.12.013 | es_ES |
| dc.description.references | Hsiao, V., Swaminathan, A., & Murray, R. M. (2018). Control Theory for Synthetic Biology: Recent Advances in System Characterization, Control Design, and Controller Implementation for Synthetic Biology. IEEE Control Systems, 38(3), 32-62. doi:10.1109/mcs.2018.2810459 | es_ES |
| dc.description.references | Huyett, L. M., Dassau, E., Zisser, H. C., & Doyle, F. J. (2018). Glucose Sensor Dynamics and the Artificial Pancreas: The Impact of Lag on Sensor Measurement and Controller Performance. IEEE Control Systems, 38(1), 30-46. doi:10.1109/mcs.2017.2766322 | es_ES |
| dc.description.references | Johnson, A. O., Gonzalez-Villanueva, M., Wong, L., Steinbüchel, A., Tee, K. L., Xu, P., & Wong, T. S. (2017). Design and application of genetically-encoded malonyl-CoA biosensors for metabolic engineering of microbial cell factories. Metabolic Engineering, 44, 253-264. doi:10.1016/j.ymben.2017.10.011 | es_ES |
| dc.description.references | Juminaga, D., Baidoo, E. E. K., Redding-Johanson, A. M., Batth, T. S., Burd, H., Mukhopadhyay, A., … Keasling, J. D. (2011). Modular Engineering of l-Tyrosine Production in Escherichia coli. Applied and Environmental Microbiology, 78(1), 89-98. doi:10.1128/aem.06017-11 | es_ES |
| dc.description.references | Koch, M., Pandi, A., Delépine, B., & Faulon, J.-L. (2018). A dataset of small molecules triggering transcriptional and translational cellular responses. Data in Brief, 17, 1374-1378. doi:10.1016/j.dib.2018.02.061 | es_ES |
| dc.description.references | LEONARD, E., YAN, Y., & KOFFAS, M. (2006). Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metabolic Engineering, 8(2), 172-181. doi:10.1016/j.ymben.2005.11.001 | es_ES |
| dc.description.references | Lin, J.-L., Wagner, J. M., & Alper, H. S. (2017). Enabling tools for high-throughput detection of metabolites: Metabolic engineering and directed evolution applications. Biotechnology Advances, 35(8), 950-970. doi:10.1016/j.biotechadv.2017.07.005 | es_ES |
| dc.description.references | Liu, D., Mannan, A. A., Han, Y., Oyarzún, D. A., & Zhang, F. (2018). Dynamic metabolic control: towards precision engineering of metabolism. Journal of Industrial Microbiology and Biotechnology, 45(7), 535-543. doi:10.1007/s10295-018-2013-9 | es_ES |
| dc.description.references | Liu, D., Xiao, Y., Evans, B. S., & Zhang, F. (2014). Negative Feedback Regulation of Fatty Acid Production Based on a Malonyl-CoA Sensor–Actuator. ACS Synthetic Biology, 4(2), 132-140. doi:10.1021/sb400158w | es_ES |
| dc.description.references | Liu, D., & Zhang, F. (2018). Metabolic Feedback Circuits Provide Rapid Control of Metabolite Dynamics. ACS Synthetic Biology, 7(2), 347-356. doi:10.1021/acssynbio.7b00342 | es_ES |
| dc.description.references | Liu, L., Shan, S., Zhang, K., Ning, Z.-Q., Lu, X.-P., & Cheng, Y.-Y. (2008). Naringenin and hesperetin, two flavonoids derived fromCitrus aurantiumup-regulate transcription of adiponectin. Phytotherapy Research, 22(10), 1400-1403. doi:10.1002/ptr.2504 | es_ES |
| dc.description.references | Mahr, R., & Frunzke, J. (2015). Transcription factor-based biosensors in biotechnology: current state and future prospects. Applied Microbiology and Biotechnology, 100(1), 79-90. doi:10.1007/s00253-015-7090-3 | es_ES |
| dc.description.references | Mannan, A. A., Liu, D., Zhang, F., & Oyarzún, D. A. (2017). Fundamental Design Principles for Transcription-Factor-Based Metabolite Biosensors. ACS Synthetic Biology, 6(10), 1851-1859. doi:10.1021/acssynbio.7b00172 | es_ES |
| dc.description.references | McKeague, M., Wong, R. S., & Smolke, C. D. (2016). Opportunities in the design and application of RNA for gene expression control. Nucleic Acids Research, 44(7), 2987-2999. doi:10.1093/nar/gkw151 | es_ES |
| dc.description.references | Nielsen, A. A. K., Der, B. S., Shin, J., Vaidyanathan, P., Paralanov, V., Strychalski, E. A., … Voigt, C. A. (2016). Genetic circuit design automation. Science, 352(6281), aac7341-aac7341. doi:10.1126/science.aac7341 | es_ES |
| dc.description.references | Nikolados, E.-M., Weiße, A. Y., Ceroni, F., & Oyarzún, D. A. (2019). Growth Defects and Loss-of-Function in Synthetic Gene Circuits. ACS Synthetic Biology, 8(6), 1231-1240. doi:10.1021/acssynbio.8b00531 | es_ES |
| dc.description.references | De Paepe, B., Maertens, J., Vanholme, B., & De Mey, M. (2018). Modularization and Response Curve Engineering of a Naringenin-Responsive Transcriptional Biosensor. ACS Synthetic Biology, 7(5), 1303-1314. doi:10.1021/acssynbio.7b00419 | es_ES |
| dc.description.references | Rahigude, A., Bhutada, P., Kaulaskar, S., Aswar, M., & Otari, K. (2012). Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience, 226, 62-72. doi:10.1016/j.neuroscience.2012.09.026 | es_ES |
| dc.description.references | Rhodius, V. A., Segall‐Shapiro, T. H., Sharon, B. D., Ghodasara, A., Orlova, E., Tabakh, H., … Voigt, C. A. (2013). Design of orthogonal genetic switches based on a crosstalk map of σs, anti‐σs, and promoters. Molecular Systems Biology, 9(1), 702. doi:10.1038/msb.2013.58 | es_ES |
| dc.description.references | Rodriguez, A., Strucko, T., Stahlhut, S. G., Kristensen, M., Svenssen, D. K., Forster, J., … Borodina, I. (2017). Metabolic engineering of yeast for fermentative production of flavonoids. Bioresource Technology, 245, 1645-1654. doi:10.1016/j.biortech.2017.06.043 | es_ES |
| dc.description.references | Segall-Shapiro, T. H., Sontag, E. D., & Voigt, C. A. (2018). Engineered promoters enable constant gene expression at any copy number in bacteria. Nature Biotechnology, 36(4), 352-358. doi:10.1038/nbt.4111 | es_ES |
| dc.description.references | Shi, S., Ang, E. L., & Zhao, H. (2018). In vivo biosensors: mechanisms, development, and applications. Journal of Industrial Microbiology and Biotechnology, 45(7), 491-516. doi:10.1007/s10295-018-2004-x | es_ES |
| dc.description.references | Shopera, T., He, L., Oyetunde, T., Tang, Y. J., & Moon, T. S. (2017). Decoupling Resource-Coupled Gene Expression in Living Cells. ACS Synthetic Biology, 6(8), 1596-1604. doi:10.1021/acssynbio.7b00119 | es_ES |
| dc.description.references | Siedler, S., Stahlhut, S. G., Malla, S., Maury, J., & Neves, A. R. (2014). Novel biosensors based on flavonoid-responsive transcriptional regulators introduced into Escherichia coli. Metabolic Engineering, 21, 2-8. doi:10.1016/j.ymben.2013.10.011 | es_ES |
| dc.description.references | Snoek, T., Chaberski, E. K., Ambri, F., Kol, S., Bjørn, S. P., Pang, B., … Keasling, J. D. (2019). Evolution-guided engineering of small-molecule biosensors. Nucleic Acids Research, 48(1), e3-e3. doi:10.1093/nar/gkz954 | es_ES |
| dc.description.references | Stevens, J. T., & Carothers, J. M. (2014). Designing RNA-Based Genetic Control Systems for Efficient Production from Engineered Metabolic Pathways. ACS Synthetic Biology, 4(2), 107-115. doi:10.1021/sb400201u | es_ES |
| dc.description.references | Trantas, E., Panopoulos, N., & Ververidis, F. (2009). Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metabolic Engineering, 11(6), 355-366. doi:10.1016/j.ymben.2009.07.004 | es_ES |
| dc.description.references | Wang, R., Cress, B. F., Yang, Z., Hordines, J. C., Zhao, S., Jung, G. Y., … Koffas, M. A. G. (2019). Design and Characterization of Biosensors for the Screening of Modular Assembled Naringenin Biosynthetic Library in Saccharomyces cerevisiae. ACS Synthetic Biology, 8(9), 2121-2130. doi:10.1021/acssynbio.9b00212 | es_ES |
| dc.description.references | Wehrs, M., Tanjore, D., Eng, T., Lievense, J., Pray, T. R., & Mukhopadhyay, A. (2019). Engineering Robust Production Microbes for Large-Scale Cultivation. Trends in Microbiology, 27(6), 524-537. doi:10.1016/j.tim.2019.01.006 | es_ES |
| dc.description.references | Xu, P., Li, L., Zhang, F., Stephanopoulos, G., & Koffas, M. (2014). Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proceedings of the National Academy of Sciences, 111(31), 11299-11304. doi:10.1073/pnas.1406401111 | es_ES |
| dc.description.references | Xu, P., Ranganathan, S., Fowler, Z. L., Maranas, C. D., & Koffas, M. A. G. (2011). Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metabolic Engineering, 13(5), 578-587. doi:10.1016/j.ymben.2011.06.008 | es_ES |
| dc.description.references | Yang, Y., Lin, Y., Li, L., Linhardt, R. J., & Yan, Y. (2015). Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metabolic Engineering, 29, 217-226. doi:10.1016/j.ymben.2015.03.018 | es_ES |
| dc.description.references | Zhou, S., Lyu, Y., Li, H., Koffas, M. A. G., & Zhou, J. (2019). Fine‐tuning the (2 S )‐naringenin synthetic pathway using an iterative high‐throughput balancing strategy. Biotechnology and Bioengineering, 116(6), 1392-1404. doi:10.1002/bit.26941 | es_ES |
| dc.description.references | Zygmunt, K., Faubert, B., MacNeil, J., & Tsiani, E. (2010). Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochemical and Biophysical Research Communications, 398(2), 178-183. doi:10.1016/j.bbrc.2010.06.048 | es_ES |
| dc.subject.ods | 08.- Fomentar el crecimiento económico sostenido, inclusivo y sostenible, el empleo pleno y productivo, y el trabajo decente para todos | es_ES |