JavaScript is disabled for your browser. Some features of this site may not work without it.
Buscar en RiuNet
Listar
Mi cuenta
Estadísticas
Ayuda RiuNet
Admin. UPV
Histology of Quercus ilex fine roots during infection by Phytophthora cinnamomi
Mostrar el registro sencillo del ítem
Ficheros en el ítem
| dc.contributor.author | Redondo, Miguel Ángel
|
es_ES |
| dc.contributor.author | Pérez Sierra, Ana María
|
es_ES |
| dc.contributor.author | Abad Campos, Paloma
|
es_ES |
| dc.contributor.author | Torres, Lilian
|
es_ES |
| dc.contributor.author | Solla, Alejandro
|
es_ES |
| dc.contributor.author | Reig Armiñana, José
|
es_ES |
| dc.contributor.author | García-Breijo, Francisco-José
|
es_ES |
| dc.date.accessioned | 2016-07-26T06:53:21Z | |
| dc.date.available | 2016-07-26T06:53:21Z | |
| dc.date.issued | 2015-12 | |
| dc.identifier.issn | 0931-1890 | |
| dc.identifier.uri | http://hdl.handle.net/10251/68170 | |
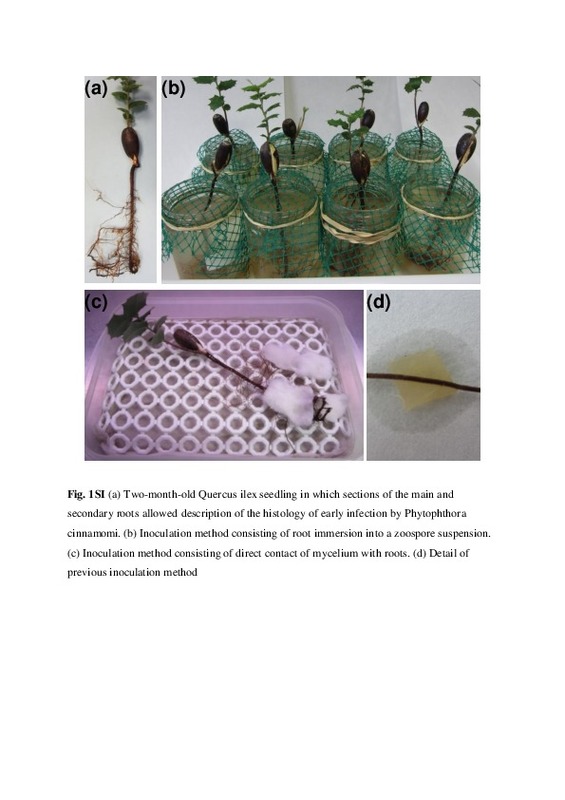
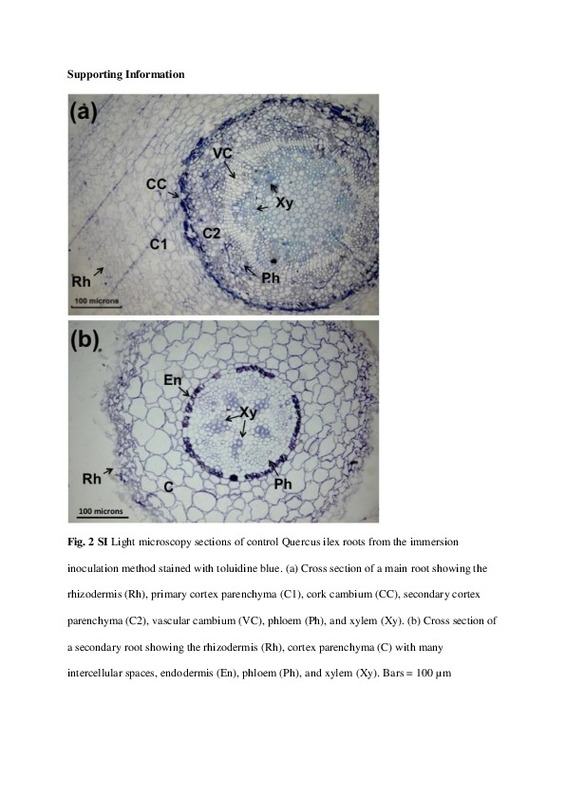
| dc.description.abstract | [EN] This study aimed to elucidate the infection process of the invasive pathogen Phytophthora cinnamomi on primary and secondary roots of 2-month-old Quercus ilex seedlings. To test if different methods of inoculation lead to different changes in the host caused by the pathogen, the root system of plants was either immersed into a suspension of P. cinnamomi zoospores, or placed in direct contact with agar plugs colonized by P. cinnamomi mycelium. Histology of root sections obtained every 24 h for 10 days revealed similar changes in the structure of cells and tissues of the host irrespective of the inoculation method used. However, the immersion method resulted in a delay in the colonization of the host, different aerial symptoms, and the formation of different reproductive structures of the pathogen. Emerging secondary and tertiary roots and sites where secondary or tertiary roots were about to emerge were identified as main entry points. Hyphae in the xylem tissues were more frequently found in secondary than in primary roots, but in both types of roots the phloem was the most important pathway of colonization. For the first time in the interaction between Q. ilex and P. cinnamomi, transmission electron microscopy was used to describe degradation of the host cell walls, pit penetration and extrahaustorial matrix. Haustoria development during intracellular growth and hyphal aggregations (stromata) caused no damage to the host cell walls indicating hemibiotrophic parasitism | es_ES |
| dc.description.sponsorship | This research was financially supported by the Project AGL2011-30438, by the Vicerrectorado de Investigacion from the Polytechnic University of Valencia, and by the "Julio Iranzo'' laboratory from the Botanic Garden of Valencia. The authors deeply appreciate the help of the staff from both institutions, particularly the valuable contribution of Nuria Cebrian Gomez. Additionally, the staff from the microscopy sections from Polytechnic University of Valencia and University of Valencia have provided us with valuable help. We are grateful to the two anonymous reviewers for the valuable comments in an earlier version of this manuscript. | en_EN |
| dc.language | Inglés | es_ES |
| dc.publisher | Springer Verlag (Germany) | es_ES |
| dc.relation | "Julio Iranzo'' laboratory from the Botanic Garden of Valencia | es_ES |
| dc.relation.ispartof | Trees - Structure and Function | es_ES |
| dc.rights | Reserva de todos los derechos | es_ES |
| dc.subject | Cell structure | es_ES |
| dc.subject | Histological alterations | es_ES |
| dc.subject | Histopathology | es_ES |
| dc.subject | Microscopy | es_ES |
| dc.subject | Pathogenesis | es_ES |
| dc.subject | Invasive pathogen | es_ES |
| dc.subject.classification | BOTANICA | es_ES |
| dc.subject.classification | PRODUCCION VEGETAL | es_ES |
| dc.subject.classification | BIOLOGIA VEGETAL | es_ES |
| dc.title | Histology of Quercus ilex fine roots during infection by Phytophthora cinnamomi | es_ES |
| dc.type | Artículo | es_ES |
| dc.identifier.doi | 10.1007/s00468-015-1275-3 | |
| dc.relation.projectID | info:eu-repo/grantAgreement/MICINN//AGL2011-30438-C02-02/ES/REGENERACION DE QUERCUS ILEX ANTE NUEVAS ESPECIES DE PHYTOPHTHORA DETECTADAS EN EL AMBITO FORESTAL/ | es_ES |
| dc.relation.projectID | info:eu-repo/grantAgreement/MICINN//AGL2011-30438-C02-01/ES/APLICACION DE TECNICAS MOLECULARES PARA VALORAR LA IMPLICACION DE PHYTOPHTHORA SPP. EN EL DECAIMIENTO DE QUERCUS ILEX/ | es_ES |
| dc.rights.accessRights | Abierto | es_ES |
| dc.contributor.affiliation | Universitat Politècnica de València. Instituto Agroforestal Mediterráneo - Institut Agroforestal Mediterrani | es_ES |
| dc.contributor.affiliation | Universitat Politècnica de València. Escuela Técnica Superior de Ingeniería Agronómica y del Medio Natural - Escola Tècnica Superior d'Enginyeria Agronòmica i del Medi Natural | es_ES |
| dc.description.bibliographicCitation | Redondo, MÁ.; Pérez Sierra, AM.; Abad Campos, P.; Torres, L.; Solla, A.; Reig Armiñana, J.; García-Breijo, F. (2015). Histology of Quercus ilex fine roots during infection by Phytophthora cinnamomi. Trees - Structure and Function. 29(6):1943-1957. https://doi.org/10.1007/s00468-015-1275-3 | es_ES |
| dc.description.accrualMethod | S | es_ES |
| dc.relation.publisherversion | https://dx.doi.org/10.1007/s00468-015-1275-3 | es_ES |
| dc.description.upvformatpinicio | 1943 | es_ES |
| dc.description.upvformatpfin | 1957 | es_ES |
| dc.type.version | info:eu-repo/semantics/publishedVersion | es_ES |
| dc.description.volume | 29 | es_ES |
| dc.description.issue | 6 | es_ES |
| dc.relation.senia | 292853 | es_ES |
| dc.identifier.eissn | 1432-2285 | |
| dc.contributor.funder | Jardín Botánico de la Universidad de Valencia | es_ES |
| dc.contributor.funder | Ministerio de Ciencia e Innovación | es_ES |
| dc.description.references | Blaschke H (1994) Decline symptoms on roots of Quercus robur. Eur J For Pathol 24:386–398. doi: 10.1111/j.1439-0329.1994.tb00832.x | es_ES |
| dc.description.references | Brummer M, Arend M, Fromm J et al (2002) Ultrastructural changes and immunocytochemical localization of the elicitin quercinin in Quercus robur L. roots infected with Phytophthora quercina. Physiol Mol Plant Pathol 61:109–120. doi: 10.1006/pmpp.2002.0419 | es_ES |
| dc.description.references | Cahill DM, Weste GM, Grant BR (1986) Changes in cytokinin concentrations in xylem extrudate following Infection of Eucalyptus marginata Donn ex Sm with Phytophthora cinnamomi Rands. Plant Physiol 81:1103–1109 | es_ES |
| dc.description.references | Cahill D, Legge B, Weste GM (1989) Cellular and histological changes induced by Phytophthora cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology 79:417–424. doi: 10.1094/Phyto-79-417 | es_ES |
| dc.description.references | Casano LM, del Campo EM, García-Breijo FJ et al (2011) Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition? Environ Microbiol 13:806–818. doi: 10.1111/j.1462-2920.2010.02386.x | es_ES |
| dc.description.references | Corcobado T, Cubera E, Pérez-Sierra A et al (2010) First report of Phytophthora gonapodyides involved in the decline of Quercus ilex in xeric conditions in Spain. New Dis Rep 22:33 | es_ES |
| dc.description.references | Corcobado T, Cubera E, Moreno G, Solla A (2013) Quercus ilex forests are influenced by annual variations in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi. Agric For Meteorol 169:92–99. doi: 10.1016/j.agrformet.2012.09.017 | es_ES |
| dc.description.references | Corcobado T, Cubera E, Juárez E et al (2014) Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric For Meteorol 192–193:1–8. doi: 10.1016/j.agrformet.2014.02.007 | es_ES |
| dc.description.references | Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013a) Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biol 117:112–123. doi: 10.1016/j.funbio.2012.12.004 | es_ES |
| dc.description.references | Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013b) Assessment of Australian native annual/herbaceous perennial plant species as asymptomatic or symptomatic hosts of Phytophthora cinnamomi under controlled conditions. For Pathol 43:245–251. doi: 10.1111/efp.12027 | es_ES |
| dc.description.references | Cubera E, Moreno G, Solla A, Madeira M (2012) Root system of Quercus suber L. seedlings in response to herbaceous competition and different watering and fertilisation regimes. Agrofor Syst 85:205–214. doi: 10.1007/s10457-012-9492-x | es_ES |
| dc.description.references | Dalio RJD, Fleischmann F, Humez M, Osswald W (2014) Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS One 9:e87860. doi: 10.1371/journal.pone.0087860 | es_ES |
| dc.description.references | de Camilo-Alves C, de Sampaio P, da Clara MIE, de Almeida Ribeiro NMC (2013) Decline of mediterranean oak trees and its association with Phytophthora cinnamomi: a review. Eur J For Res 132:411–432. doi: 10.1007/s10342-013-0688-z | es_ES |
| dc.description.references | Fahn A (1990) Plant anatomy. Pergamon Press, Oxford | es_ES |
| dc.description.references | Hansen EM, Parke JL, Sutton W (2005) Susceptibility of Oregon forest trees and shrubs to Phytophthora ramorum: a comparison of artificial inoculation and natural infection. Plant Dis 89:63–70. doi: 10.1094/PD-89-0063 | es_ES |
| dc.description.references | Haque MMU, Diez JJ (2012) Susceptibility of common alder (Alnus glutinosa) seeds and seedlings to Phytophthora alni and other Phytophthora species. For Syst 21:313–322. doi: 10.5424/fs/2012212-02267 | es_ES |
| dc.description.references | Hardham AR (2001) The cell biology behind Phytophthora pathogenicity. Australas Plant Pathol 30:91–98. doi: 10.1071/AP01006 | es_ES |
| dc.description.references | Hardham AR (2005) Phytophthora cinnamomi. Mol Plant Pathol 6:589–604. doi: 10.1111/j.1364-3703.2005.00308.x | es_ES |
| dc.description.references | Hatakka A (2005) Biodegradation of lignin. Biopolym Online. doi: 10.1002/3527600035.bpol1005 | es_ES |
| dc.description.references | Horta M, Caetano P, Medeira C et al (2010) Involvement of the β-cinnamomin elicitin in infection and colonisation of cork oak roots by Phytophthora cinnamomi. Eur J Plant Pathol 127:427–436. doi: 10.1007/s10658-010-9609-x | es_ES |
| dc.description.references | Judelson HS, Blanco FA (2005) The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol 3:47–58. doi: 10.1038/nrmicro1064 | es_ES |
| dc.description.references | Jung T, Cooke DEL, Blaschke H et al (1999) Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol Res 103:785–798. doi: 10.1017/S0953756298007734 | es_ES |
| dc.description.references | Jung T, Colquhoun IJ, Hardy GESJ (2013) New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in Western Australia. For Pathol 43:266–288. doi: 10.1111/efp.12025 | es_ES |
| dc.description.references | Kamoun S, Furzer O, Jones JDG et al (2015) The top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol 16:413–434. doi: 10.1111/mpp.12190 | es_ES |
| dc.description.references | Laliberté E, Lambers H, Burgess TI, Wright SJ (2015) Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. N Phytol 206:507–521. doi: 10.1111/nph.13203 | es_ES |
| dc.description.references | Linaldeddu BT, Scanu B, Maddau L, Franceschini A (2014) Diplodia corticola and Phytophthora cinnamomi: the main pathogens involved in holm oak decline on Caprera Island (Italy). For Pathol 44:191–200. doi: 10.1111/efp.12081 | es_ES |
| dc.description.references | Lu Y-J, Schornack S, Spallek T et al (2012) Patterns of plant subcellular responses to successful oomycete infections reveal differences in host cell reprogramming and endocytic trafficking. Cell Microbiol 14:682–697. doi: 10.1111/j.1462-5822.2012.01751.x | es_ES |
| dc.description.references | Martín-García J, Solla A, Corcobado T et al (2015) Influence of temperature on germination of Quercus ilex in Phytophthora cinnamomi, P. gonapodyides, P. quercina and P. psychrophila infested soils. For Pathol 45:215–223. doi: 10.1111/efp.12159 | es_ES |
| dc.description.references | Maurel M, Robin C, Capron G, Desprez-Loustau M-L (2001) Effects of root damage associated with Phytophthora cinnamomi on water relations, biomass accumulation, mineral nutrition and vulnerability to water deficit of five oak and chestnut species. For Pathol 31:353–369. doi: 10.1046/j.1439-0329.2001.00258.x | es_ES |
| dc.description.references | McConnell ME, Balci Y (2015) Fine root dynamics of oak saplings in response to Phytophthora cinnamomi infection under different temperatures and durations. For Pathol 45:155–164. doi: 10.1111/efp.12150 | es_ES |
| dc.description.references | Mullendore DL, Windt CW, As HV, Knoblauch M (2010) Sieve tube geometry in relation to phloem flow. Plant Cell Online 22:579–593. doi: 10.1105/tpc.109.070094 | es_ES |
| dc.description.references | O’Gara E, Howard K, McComb J et al (2015) Penetration of suberized periderm of a woody host by Phytophthora cinnamomi. Plant Pathol 64:207–215. doi: 10.1111/ppa.12244 | es_ES |
| dc.description.references | Oh E, Hansen EM (2007) Histopathology of infection and colonization of susceptible and resistant Port-Orford-Cedar by Phytophthora lateralis. Phytopathology 97:684–693. doi: 10.1094/PHYTO-97-6-0684 | es_ES |
| dc.description.references | Oßwald W, Fleischmann F, Rigling D et al (2014) Strategies of attack and defence in woody plant–Phytophthora interactions. For Pathol 44:169–190. doi: 10.1111/efp.12096 | es_ES |
| dc.description.references | Pérez-Sierra A, López-García C, León M et al (2013) Previously unrecorded low-temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in eastern Spain. For Pathol 43:331–339. doi: 10.1111/efp.12037 | es_ES |
| dc.description.references | Pulido F, McCreary D, Cañellas I et al (2013) Oak regeneration: ecological dynamics and restoration techniques. In: Campos P, Huntsinger L, Oviedo Pro JL (eds) Mediterranean oak woodland working landscapes. Springer, Dordrecht, pp 123–144 | es_ES |
| dc.description.references | Robin C, Capron G, Desprez-Loustau ML (2001) Root infection by Phytophthora cinnamomi in seedlings of three oak species. Plant Pathol 50:708–716. doi: 10.1046/j.1365-3059.2001.00643.x | es_ES |
| dc.description.references | Rodríguez-Molina MC, Torres-Vila LM, Blanco-Santos A et al (2002) Viability of holm and cork oak seedlings from acorns sown in soils naturally infected with Phytophthora cinnamomi. For Pathol 32:365–372. doi: 10.1046/j.1439-0329.2002.00297.x | es_ES |
| dc.description.references | Ruiz de la Torre J (2006) Flora mayor. Organismo Autónomo Parques Nacionales, Dirección General para la Biodiversidad, Madrid | es_ES |
| dc.description.references | Ruiz Gómez FJ, Navarro-Cerrillo RM, Sánchez-Cuesta R, Pérez-de-Luque A (2015) Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathol 64:605–616. doi: 10.1111/ppa.12310 | es_ES |
| dc.description.references | Ruiz-Gómez FJ, Sánchez-Cuesta R, Navarro-Cerrillo RM, Pérez-de-Luque A (2012) A method to quantify infection and colonization of holm oak (Quercus ilex) roots by Phytophthora cinnamomi. Plant Methods 8:39. doi: 10.1186/1746-4811-8-39 | es_ES |
| dc.description.references | Rytkönen A, Lilja A, Werres S et al (2013) Infectivity, survival and pathology of Finnish strains of Phytophthora plurivora and Ph. pini in Norway spruce. Scand J For Res 28:307–318. doi: 10.1080/02827581.2012.756926 | es_ES |
| dc.description.references | Tsao PH (1990) Why many phytophthora root rots and crown rots of tree and horticultural crops remain undetected? EPPO Bull 20:11–17. doi: 10.1111/j.1365-2338.1990.tb01174.x | es_ES |
| dc.description.references | Underwood W (2012) The plant cell wall: a dynamic barrier against pathogen invasion. Front Plant Sci 3:85. doi: 10.3389/fpls.2012.00085 | es_ES |
| dc.description.references | Willetts HJ (1997) Morphology, development and evolution of stromata/sclerotia and macroconidia of the Sclerotiniaceae. Mycol Res 101:939–952. doi: 10.1017/S0953756297003559 | es_ES |








![[Cerrado]](/themes/UPV/images/candado.png)

